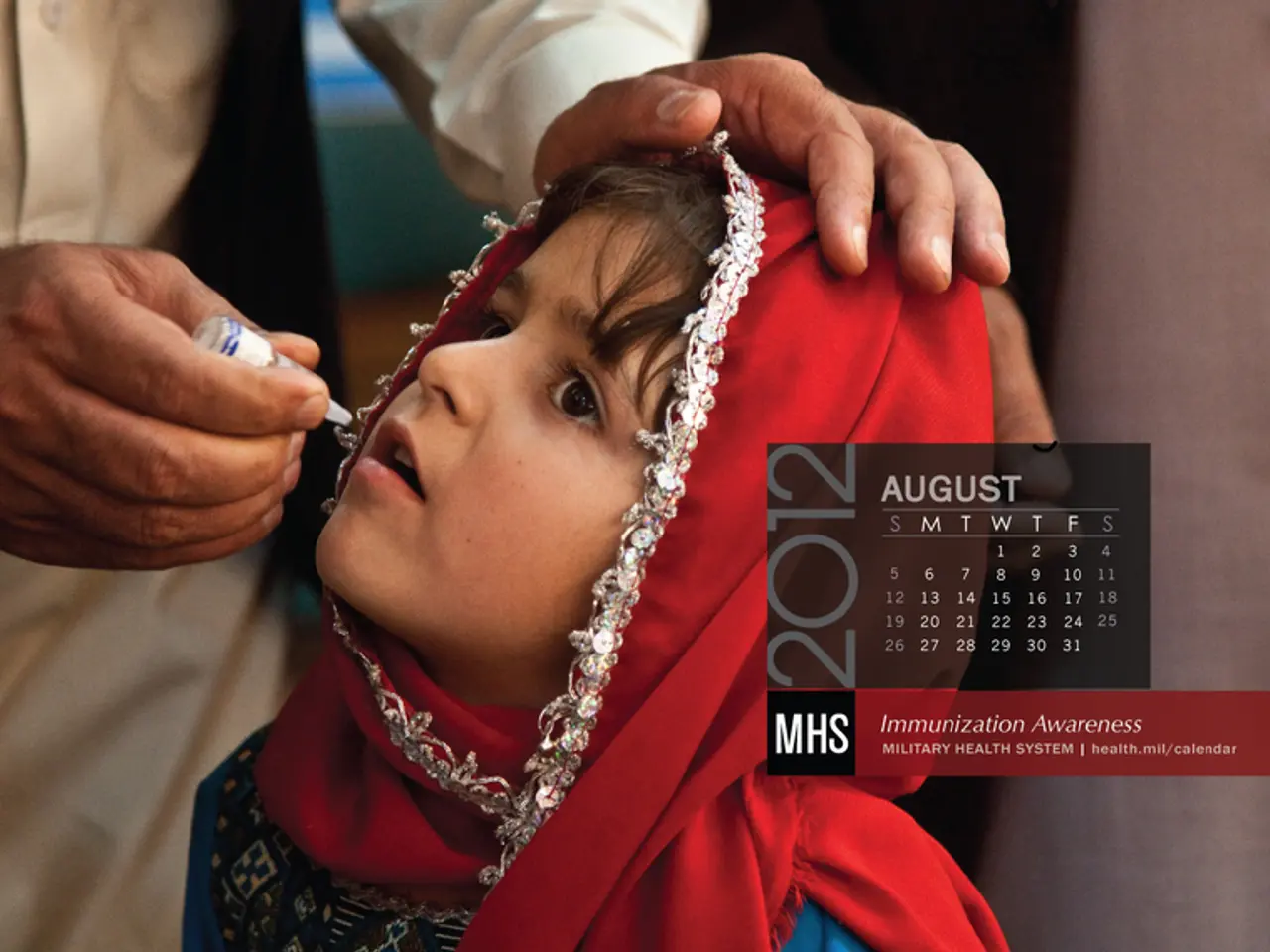
Voting concludes with CDC advisers opposing a combined vaccine for young children, and a delayed decision on a newborn hepatitis B inoculation.
The CDC's Advisory Committee on Immunization Practices (ACIP) has delayed a vote on hepatitis B vaccines for newborns until Friday, sparking concerns among some experts. The committee is expected to make a decision about changing the recommendation to delay the hepatitis B vaccination for newborns to age 4 during their meeting scheduled for September 18–19, 2025.
The forthcoming ACIP vote on hepatitis B shots could do damage, according to some experts, by missing the most highly vulnerable window for protection of infants. Hepatitis B is highly contagious and can cause chronic infections in nearly 90% of infected infants, increasing the risk of liver scarring, cancer, or the need for a liver transplant later in life.
Since 1991, the CDC has recommended that babies get their first dose of a hepatitis B vaccine soon after birth. This recommendation has been a cornerstone of the Vaccines for Children (VFC) program, which provides access for low-income children. However, during a recent meeting, eight members of the ACIP voted not to change the recommendation for the VFC program. One member abstained, and three voted yes.
The delay in the vote has raised questions about the committee's decision-making process. Some members felt that delaying a baby's vaccination ignores the possibility of transmission outside of the mother. Hepatitis B can potentially be transmitted through contact with tiny amounts of blood on razors, toothbrushes, or bites from another child in day care. The virus can survive on surfaces for up to seven days.
Dr. Evelyn Griffin, an obstetrician-gynecologist in Louisiana, felt that the birth dose of the hepatitis B vaccine is a matter of informed consent. She argued that the benefits of the vaccine outweigh the risks, and that parents should be able to make an informed decision about their child's health.
The ACIP's recommendations shape doctors' guidance to patients, state vaccine policy, the Vaccines for Children program, and insurance coverage. The committee has undergone significant changes in the past few months, with many members being replaced by appointees chosen by US Health and Human Services Secretary Robert F. Kennedy Jr.
In a related decision, the ACIP voted 8-3 that the combined measles, mumps, rubella, and varicella (MMRV) vaccine is not recommended before age 4. Children in this age group should receive the measles, mumps, and rubella, or MMR, vaccine separately from the varicella vaccine.
Delaying a baby's vaccination increases the risk they won't get their shot at all. The CDC estimates that about 1 in 4 children who are infected with hepatitis B will die prematurely from their infections. The ACIP is scheduled to continue its meeting on Friday with a discussion and vote on Covid-19 vaccines for all age groups, in addition to the vote on hepatitis B shots.
Read also:
- Countries initiate efforts to prohibit smoking within vehicles
- Backed by Scientific Evidence, 11 Strong Arguments for Almond Appreciation
- Administration's effort to dismiss thousands of Health and Human Services employees denied by the court
- Funds amounting to approximately 1.17 million euros designated for local meetings regarding nursing practices