Unforeseen chaos marks initial CDC vaccine panel session, causing postponement of hepatitis B vaccine decision and prompting vote on MMRV vaccine instead
In a surprising turn of events, Jim O'Neill, the acting director of the Centers for Disease Control and Prevention (CDC), has announced a delay in the vote on changes to the newborn hepatitis B vaccine. This decision comes after the CDC's vaccine advisers voted 11-1 to postpone a decision on the matter.
The advisory group in question, the Advisory Committee on Immunization Practices (ACIP) of the CDC, had planned to consider a new recommendation that would wait to give newborns a dose of the hepatitis B vaccine until they are at least a month old. However, the vote on election day has brought about a pause in this process.
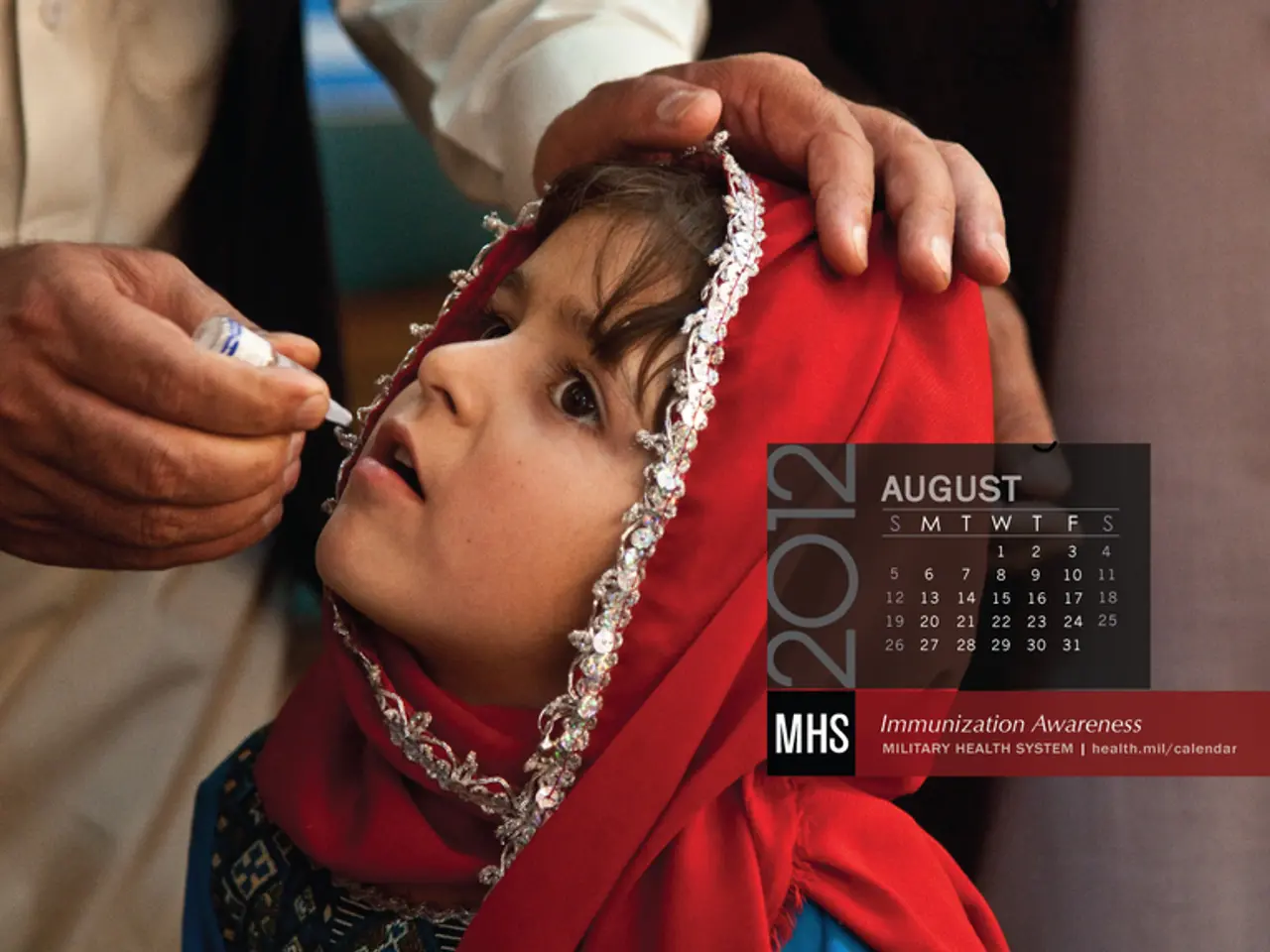
The current practice, which has been recommended in the United States since 1991, involves administering the hepatitis B vaccine to babies at birth, usually before they leave the hospital. This practice has been instrumental in significantly reducing hepatitis B infections in infants. Since its implementation, the number of hepatitis B infections in infants has dropped from an average of 18,000 per year to around 20 reported cases of hepatitis B in babies per year now.
It's important to note that children infected with hepatitis B nearly always develop long-term infections which can damage the liver. Therefore, the importance of a vaccination program against this disease cannot be overstated.
Dr. Jason Goldman, president of the American College of Physicians, expressed concern about the conflicting votes regarding the hepatitis B vaccine. However, he also suggested that the lack of a unanimous decision might indicate a lack of data or evidence to challenge the current standing. Goldman implied that there is no associated harm with the current standing of the vaccine.
The Health and Human Services (HHS) will examine all insurance coverage implications following the ACIP recommendation before a final decision is made. The vote to delay the decision on the hepatitis B vaccine for newborns is a significant development in public health policy, and we will continue to update this story as more information becomes available.
Read also:
- Countries initiate efforts to prohibit smoking within vehicles
- Backed by Scientific Evidence, 11 Strong Arguments for Almond Appreciation
- Administration's effort to dismiss thousands of Health and Human Services employees denied by the court
- Funds amounting to approximately 1.17 million euros designated for local meetings regarding nursing practices